Biomedical Informatics and Smart Healthcare
ISSN: 3068-5524 (Online)
Email: [email protected]


Research has identified a negative correlation between amygdala and ventromedial prefrontal cortex (vmPFC) functional connectivity, which plays a crucial role in emotion regulation. This relationship is influenced by factors such as inflammation, development, and neurofeedback training. Mehta et al. [1] found that increased inflammation predicted decreased amygdala-vmPFC connectivity in depressed patients, correlating with anxiety symptoms. Motzkin et al. [2] demonstrated that vmPFC lesions led to heightened amygdala activity, supporting its regulatory role. Gee et al. [3] observed a developmental shift from positive to negative amygdala-prefrontal connectivity during the transition to adolescence, potentially explaining improvements in emotional regulation. Paret et al. [4] showed that voluntary amygdala down-regulation through fMRI neurofeedback increased amygdala-vmPFC connectivity, with a bottom-up information flow from amygdala to vmPFC. These findings highlight the complex interplay between amygdala and vmPFC in emotional processing and regulation, offering insights into potential therapeutic approaches for mood and anxiety disorders.
Understanding the neural dynamics among brain regions has provided neuroscientists with valuable insights into constructing a robust model for how the brain regulates emotion and makes economic decisions [5, 6]. Such study is especially crucial in the fields of affective neuroscience and neuroeconomics, notably the investigation of the relationship between vmPFC and amygdala in rational adults [7, 8, 2, 9, 10]. These literatures have shown a negative functional connectivity between these two regions, indicating a negative temporal correlation across the two distant regions. However, correlation in neuroscience alone does not necessarily prove causation [11]. Most of these studies primarily rely on using static correlation, which only records activity at a specific moment in time, to identify the functional connectivity between regions.
Other studies, however, have attempted to explore causality between vmPFC and amygdala using Granger causality analysis (GCA). For example, Seth et al. [13] used GCA to interpret Functional Magnetic Resonance Imaging (fMRI) data, revealing its potential for inferring causal interaction in neural networks. Still, these analysis methods rely on low-temporal, high-spatial resolution data from fMRI, which might undermine the rapid, dynamic causal interplay between regions (in this case, vmPFC and amygdala) involved in emotion regulation and economic decision-making [12].
As Dosenbach et al. [14] suggested, a multidimensional approach is essential to robustly capture the complexity of neural interactions. Yet, most studies still tend to utilize one or two methods for analysis. For instance, Gold et al. [15] concentrated on connectivity but failed to examine signal complexity or causal relationships between vmPFC and amygdala, limiting their understanding of the vmPFC-amygdala interaction. Hence, in this paper, a multidimensional approach is utilized to study amygdala-vmPFC interaction from EEG data.
First, we move beyond static connectivity by applying time-lagged correlation to explore how these regions influence each other over time, a technique not commonly used in previous research. Second, we employ GCA to investigate predictive relationships, something often missing in EEG-based studies of these regions. Third, the entropy metric is implemented into our analysis to offer insights into the complexity of signals in both regions. This provides a unique and dynamic perspective on how the amygdala and vmPFC might handle different levels of information processing during tasks involving emotion regulation or decision-making. Lastly, by combining these techniques, we offer a holistic analysis that moves beyond what single-method studies have achieved, filling critical gaps in literature.
In other words, the following questions are addressed within this paper:
To what extent are the amygdala and vmPFC functionally connected?
Does the interaction between amygdala and vmPFC abide with a time-lagged pattern?
Which brain region displays more complex activities?
Can neural activity of one region predict that of another region?
In essence, our solution not only maps the connectivity between the amygdala and vmPFC but also explores the temporal dynamics, predictability, and complexity of their interactions in a way that previous studies have largely overlooked.
The dataset used in this study comprises intracranial EEG (iEEG) and neuronal data recorded from the amygdalae of nine human subjects during exposure to emotionally aversive and neutral visual stimuli [16]. Data collection was performed in a controlled clinical setting, and the dataset was obtained from OpenNeuro in the BIDS standard format.
The electrophysiological data include:
Intracranial EEG (iEEG): High temporal resolution signals from electrodes implanted in the amygdala. Spectral analysis validated the iEEG signals.
Neuronal Spike Times and Waveforms: Neuronal firing data, including spike sorting quality metrics.
Metadata: Task-related information, subject details, session information, and electrode specifications.
This dataset is publicly available through OpenNeuro under the title "Dataset of neurons and intracranial EEG from human amygdala during aversive dynamic visual stimulation" [16]. The dataset underwent rigorous technical validation, including:
Spike sorting quality assessment for neuronal firing data.
Spectral analysis of iEEG signals to ensure data integrity.
This comprehensive dataset provides a unique opportunity to study the amygdala's role in processing emotional stimuli at both macroscopic and microscopic levels. It was instrumental in analyzing the functional connectivity and causal interactions between the amygdala and vmPFC in the present study.
Functional connectivity demonstrates the temporal relationship between spatially distant brain regions. Functional connectivity, which is usually determined through EEG and fMRI [17]. In our study, we used the Pearson correlation coefficient to examine functional connectivity between the neural activities of amygdala and vmPFC, as it indicates how synchronized these two regions are. However, Rousselet and Pernet [20] have pointed out that the Pearson correlation might be an unrobust metric due to its high sensitivity to outliers. This indicates that relying solely on Pearson correlation can yield unreliable conclusions for understanding the relation between brain and behavior. Despite this limitation, we included the Pearson correlation as an initial measure, utilizing it as a preliminary reference to support further validation of functional connectivity between the amygdala and vmPFC. This approach allows us to capture basic connectivity patterns, potentially helping future research to refine more robust analytical methods.
Time-lagged correlation measures how one variable temporally influences another, allowing us to examine the stability of the Pearson correlation coefficient in representing functional connectivity. In this study, time-lagged correlation is used to test the robustness of the Pearson correlation by assessing whether the observed causality between the amygdala and vmPFC is significant over time. While this approach can hint at causation across functionally connected regions, it falls short of providing conclusive evidence of temporal causation. According to Rykhlevskaia et al. [19], time-lagged correlation does not provide adequate information to prove the existence of such temporal causation. To address this drawback, we used Granger Causality Analysis (GCA) to corroborate the directional influences between the amygdala and vmPFC.
Granger Causality Analysis (GCA) is a statistical method used to evaluate the predictive relationship between two time-series variables by assessing whether the past activity of one variable can predict the future activity of another. In this study, GCA was selected as the primary method for exploring the directional influence between the amygdala and the ventromedial prefrontal cortex (vmPFC). By leveraging the high temporal resolution of intracranial EEG (iEEG) signals, GCA provides insights into how these regions dynamically influence each other during emotionally aversive tasks [13].
SSR-Based F-Test: Evaluates whether adding the past activity of one region significantly improves the prediction of another's future activity. While useful, it primarily identifies reduction in residual error but does not explicitly model temporal influence.
SSR-Based Chi-Square Test: Measures variance explained by including lagged predictors but lacks the time-lag-specific modeling offered by GCA.
Likelihood Ratio Test: Compares the likelihood of the data under two models (with and without lagged predictors) but does not address the directionality of influence.
Parameter F-Test: Assesses individual regression coefficients for significance but is less suited for identifying overall causal patterns in a time-lagged context.
To quantify the complexity and randomness of neural activity in the amygdala and vmPFC, we employed Shannon Entropy, a widely used metric for assessing signal variability. Based on Carhart-Harris's entropy brain hypothesis, entropy provides a mathematical framework to evaluate the degree of randomness within a system, which in this context reflects the unpredictability of neural signals in each brain region.
Signal Input: The entropy calculation was performed on pre-processed iEEG signals recorded from the amygdala and vmPFC. Pre-processing steps included noise and artifact removal.
Time Windows: Entropy was computed using a sliding window approach (e.g., 500 ms) to capture temporal variations.
Probability Distribution: Signal amplitudes within each time window were discretized into bins. The probability for each bin was calculated.
Shannon Entropy Formula:
Regional Entropy: Separate entropy values were computed for the amygdala and vmPFC.
Temporal Trends: Changes in entropy over time were analyzed to assess variability.
Complexity Analysis: Comparing entropy across regions highlighted differences in signal unpredictability.
Dynamic Interactions: Aligned temporal entropy patterns helped infer excitatory or inhibitory interactions.
Causal Insights: Synchrony in entropy fluctuations hinted at potential directional influence.
The Pearson correlation analysis revealed a significant negative correlation of (-value < 0.05) between the amygdala and the vmPFC, as shown in Figure 1. This suggests an inverse relationship: when one region becomes more active, the other tends to become less active. Such an inhibitory interaction supports the vmPFC's role in regulating the amygdala's emotional reactivity.
This negative correlation confirms functional connectivity between the two regions, which concludes our first research objective successfully, i.e., Are these regions functionally connected?

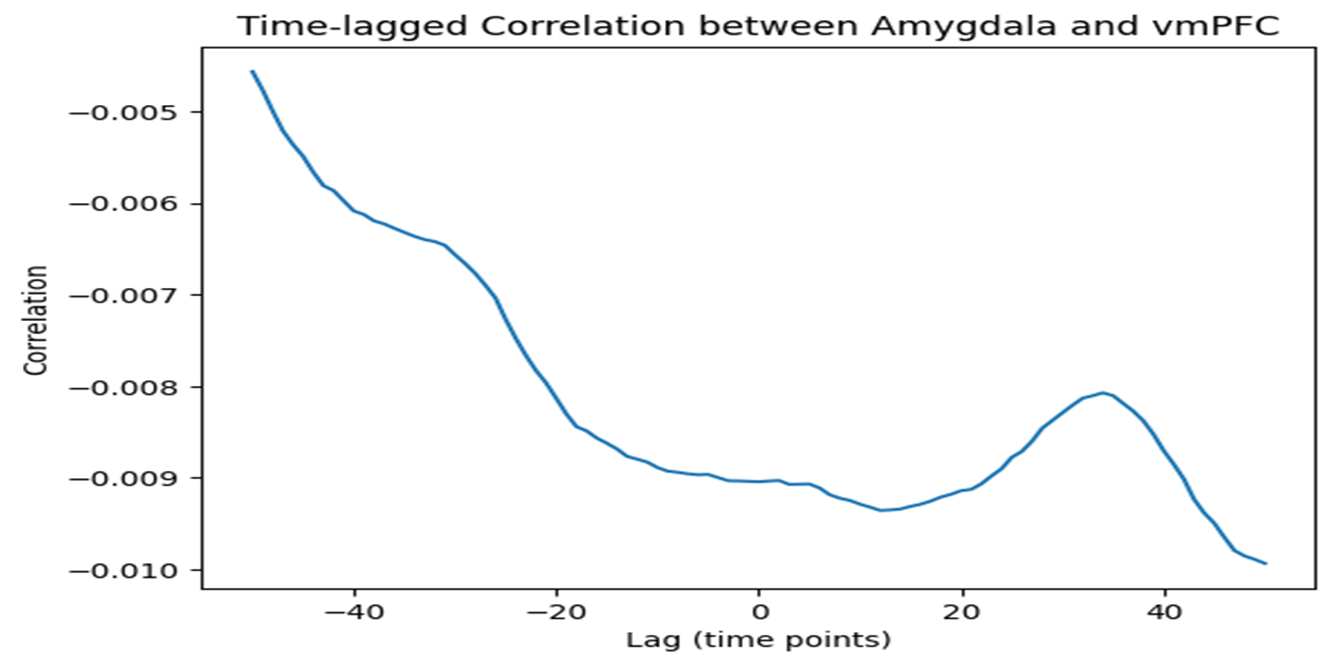
Time-lagged correlation analysis revealed the strongest negative correlation at a lag of approximately time points, indicating that the amygdala's activity leads the vmPFC by this delay, as seen in Figure 2. This temporal pattern suggests a sequential process, with the amygdala initiating neural responses and the vmPFC following to modulate emotional processing.
This time lag indicates a sequential interaction with the amygdala leading, which concludes our second research objective successfully, i.e., Do their interactions follow a time-lagged pattern?
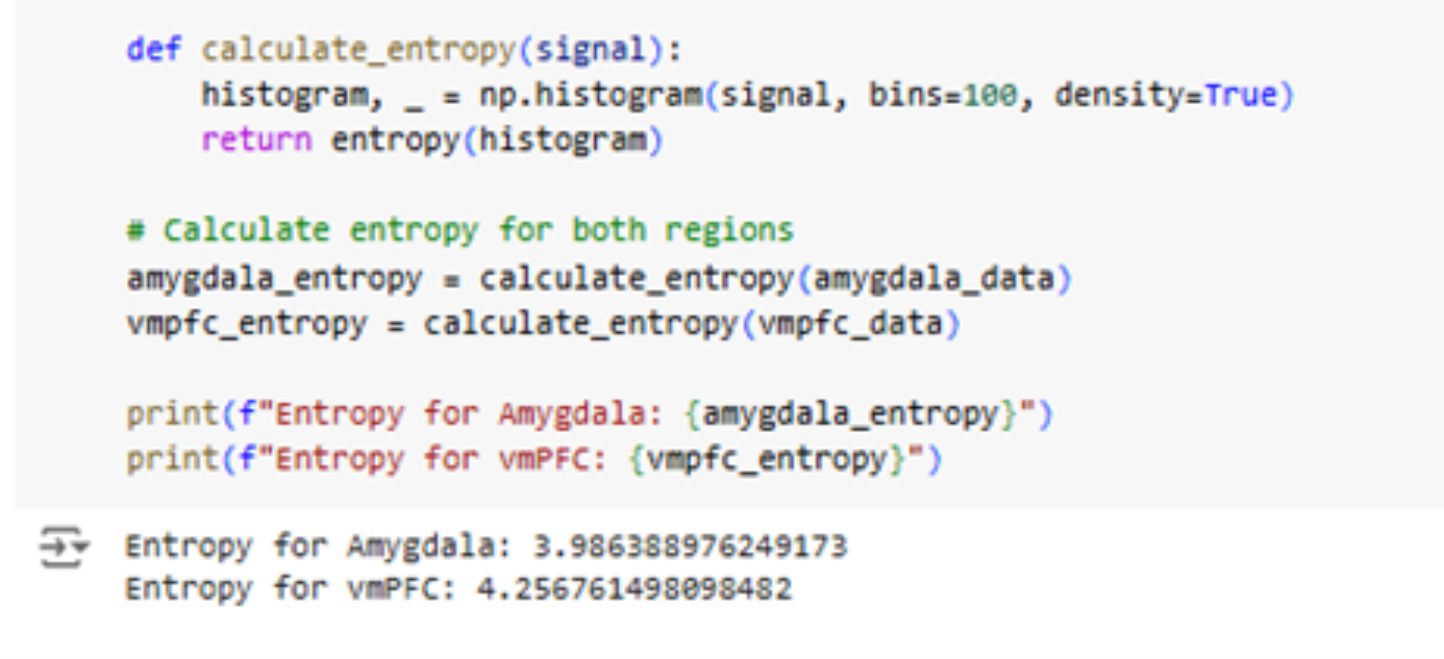
Entropy analysis revealed that the vmPFC had higher entropy () compared to the amygdala (), indicating more complex and unpredictable activity in the vmPFC, as shown in Figure 3. This aligns with its role in integrating higher-order cognitive functions, such as decision-making and emotional regulation.
These outputs conclude that the vmPFC's higher entropy reflects its more complex neural activity, which concludes our third research objective successfully, i.e., Is one region's activity more complex than the other?
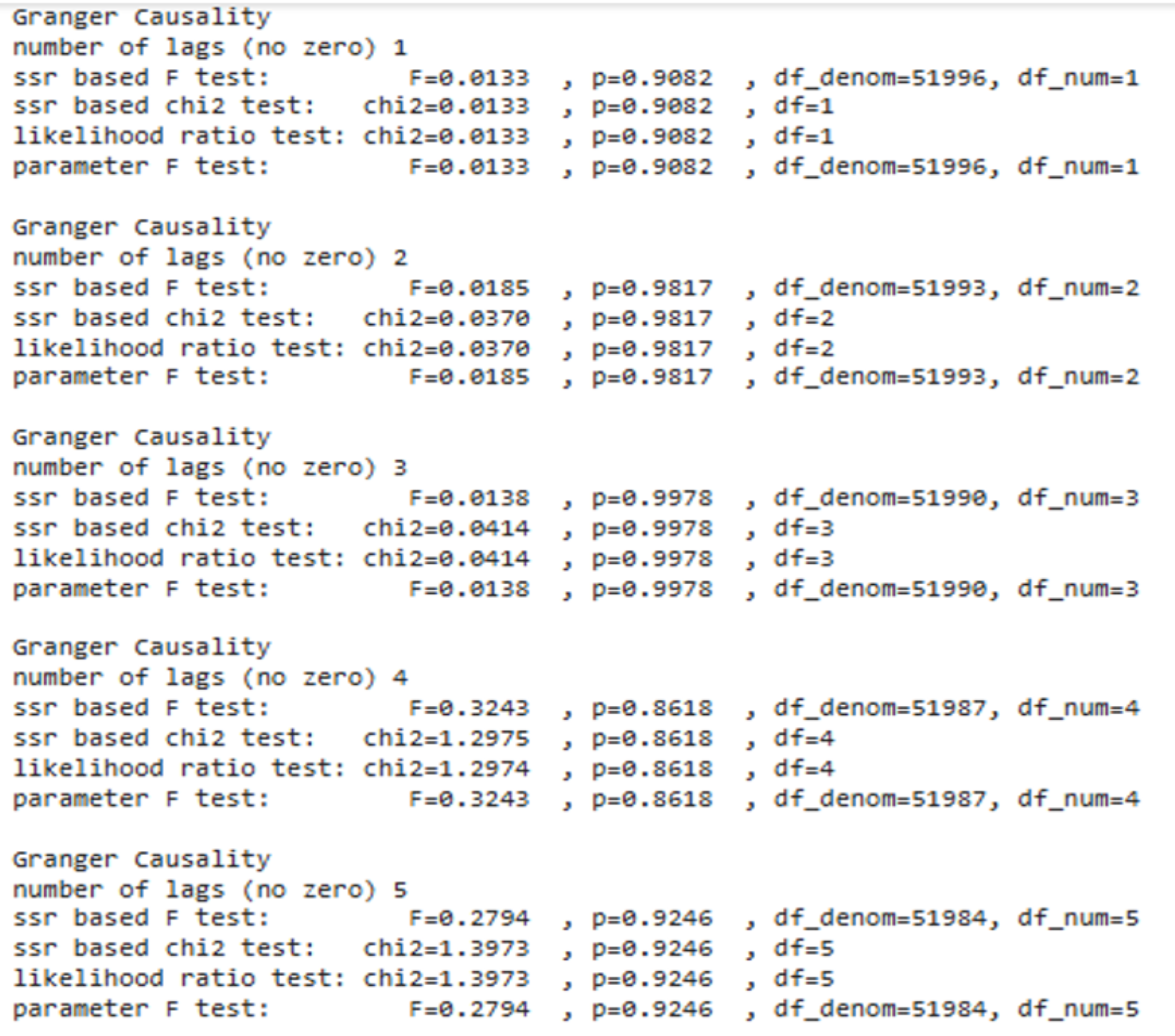
Granger causality testing did not reveal statistically significant predictive relationships between the amygdala and vmPFC (all -values > 0.05), as shown in Figure 4. This suggests that the regions' interactions may not follow a simple linear causal pattern but could involve more complex dynamics influenced by external factors or other brain regions.
These outputs conclude that the weak evidence for predictive influence between the amygdala and vmPFC suggests that their interactions may involve more complex, non-linear dynamics. This addresses our fourth research objective, i.e., Can we predict one region's activity based on the other?, by indicating that a straightforward causal relationship is unlikely in this context.
This study aimed to clarify the dynamic relationship between the amygdala and the ventromedial prefrontal cortex (vmPFC) in regulating emotions. By employing a multidimensional analysis that integrated functional connectivity, time-lagged correlation, Granger causality, and entropy metrics, we provided a nuanced understanding of how these regions interact during emotionally aversive tasks. Below, we discuss our findings in the context of prior literature and highlight their implications for neuroscience and neuroeconomics.
Consistent with previous studies [7, 21, 23], our results confirmed a significant functional connection between the amygdala and vmPFC, evidenced by a statistically significant negative correlation. This aligns with the established role of the vmPFC in inhibiting amygdala-driven emotional reactivity. However, Pearson correlation, which only captures linear relationships, may oversimplify the complexity of brain interactions, as neural communication often involves non-linear dynamics [18, 24]. This limitation underscores the need for advanced methods that account for such complexity, as functional connectivity alone cannot fully explain the causal dynamics between brain regions.
The time-lagged correlation analysis revealed a temporal delay of approximately to time points, with the amygdala leading and the vmPFC following. This finding supports the hypothesis that the amygdala initiates emotional responses, which are subsequently modulated by the vmPFC. However, Granger causality analysis provided weak evidence for a direct causal relationship between the regions, contradicting some previous findings [2]. This discrepancy may stem from GCA's inherent limitations, including its reliance on linear assumptions and sensitivity to noise in high-variance neural systems [22]. These results highlight the possibility that the amygdala-vmPFC interaction involves more intricate, non-linear dynamics than can be captured by GCA alone.
Entropy metrics introduced a novel dimension to our understanding of the amygdala-vmPFC relationship. The vmPFC exhibited higher entropy values compared to the amygdala, reflecting its greater complexity and integration of diverse neural inputs. This finding complements prior studies showing that the vmPFC's activity correlates with emotional regulation [25]. By combining entropy metrics with time-lagged correlation and functional connectivity, our analysis suggests a regulatory mechanism where the vmPFC modulates amygdala activity over time. This integrated approach offers a robust framework for exploring causality in neural systems and addresses the limitations of linear causality methods like GCA.
While our study achieved significant and novel insights into the dynamics of the amygdala-vmPFC relationship, there remain opportunities to expand upon this work. The integration of functional connectivity, time-lagged correlation, Granger causality, and entropy metrics provides a powerful and innovative framework that advances the understanding of brain region interactions. However, the complexities of neural systems and the limitations of current methodologies invite further exploration to build upon our novel findings.
This study investigated the dynamic neural relationship between the amygdala and the ventromedial prefrontal cortex (vmPFC) during emotionally aversive tasks using a multidimensional analysis framework. We confirmed a statistically significant negative functional correlation (, ), indicating inhibitory connectivity. Time-lagged correlation analysis revealed that amygdala activity precedes vmPFC response by approximately 30 time points, suggesting sequential emotional regulation. Entropy analysis showed that the vmPFC exhibited greater signal complexity (H = 4.26) than the amygdala (H = 3.98), supporting its role in higher-order cognitive processing. Granger causality analysis, however, did not detect a significant predictive relationship, implying the presence of more complex or nonlinear dynamics.
Key Contributions:
Proposed a novel, multidimensional methodology combining functional connectivity, time-lagged correlation, Granger causality, and entropy metrics on human iEEG data.
Demonstrated that vmPFC–amygdala interactions are temporally ordered and functionally connected, but not linearly causal.
Introduced entropy-based complexity analysis as a valuable metric to complement traditional connectivity and causality tools.
Provided a reproducible framework for studying emotion-related neural circuits with implications for neuroscience, affective computing, and neuroeconomics.
These findings offer a richer understanding of the amygdala-vmPFC axis and emphasize the need to move beyond linear models in neuroscience. Future work may explore nonlinear causal inference techniques, integrate multimodal data, or apply this framework to clinical populations to assess psychiatric or decision-making disorders.
 Copyright © 2025 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
Copyright © 2025 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. 
Portico
All published articles are preserved here permanently:
https://www.portico.org/publishers/icck/