Journal of Chemical Engineering and Renewable Fuels
ISSN: 3070-1058 (Online)
Email: [email protected]


 Submit Manuscript
Edit a Special Issue
Submit Manuscript
Edit a Special Issue
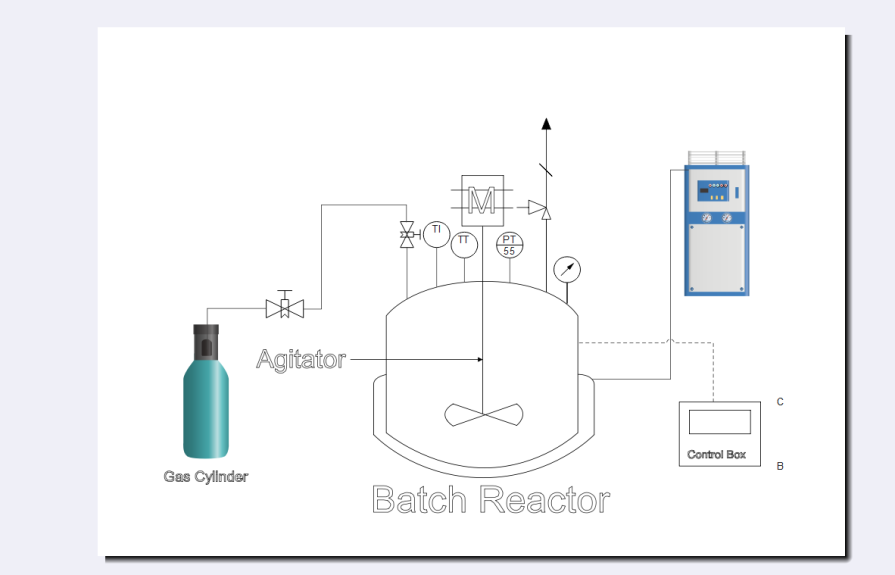
TY - JOUR AU - Pandey, Ankit AU - Nandi, Monojit AU - Dwivedi, Gaurav AU - Singh, Ajay AU - Maity, Samir Kumar AU - Poddar, Mukesh Kumar PY - 2025 DA - 2025/12/08 TI - Oxygen Insertion in Propylene to Make Propylene Oxide Over a Highly Stable and Efficient Titanium Silicate Catalyst JO - Journal of Chemical Engineering and Renewable Fuels T2 - Journal of Chemical Engineering and Renewable Fuels JF - Journal of Chemical Engineering and Renewable Fuels VL - 2 IS - 1 SP - 13 EP - 22 DO - 10.62762/JCERF.2025.147821 UR - https://www.icck.org/article/abs/JCERF.2025.147821 KW - propylene KW - propylene oxide KW - TS-1 KW - epoxidation AB - Propylene oxide (PO) is a crucial intermediate in the chemical industry, serving as a precursor for polyether polyols, propylene glycol, and various polymers. Traditional industrial PO production methods, such as the chlorohydrin and hydroperoxide routes, are hampered by significant environmental concerns and the generation of undesirable by-products. This study explores the direct transformation of propylene to PO using titanium silicate (TS-1) catalyst. The catalyst (TS-1) was synthesized and characterized by BET surface analysis, X-ray diffraction (XRD), scanning and transmission electron microscopy (SEM/TEM), and X-ray photoelectron spectroscopy (XPS). These analyses confirmed the formation of a crystalline MFI-type framework with well-dispersed titanium sites and a uniform, spherical morphology. Catalytic performance was evaluated in a batch reactor under varying conditions of temperature, pressure, hydrogen peroxide, and methanol concentrations. Under varying conditions, a maximum PO selectivity of 99.5% and a PO yield of up to 9% were achieved. The highest selectivity was obtained at lower H2O2 concentration, while the maximum yield required a higher H2O2 loading. The study also demonstrated the catalyst’s reusability and stability over multiple cycles, with minimal loss in activity. Side reactions, primarily the formation of propylene glycol and its ethers, were minimized by controlling water and hydrogen peroxide concentrations. The results highlight the advantages of TS-1, including high selectivity, environmental compatibility, and operational stability, making it a promising candidate for sustainable PO synthesis. This work provides valuable insights into the design of advanced heterogeneous catalysts for green chemical processes and addresses key challenges in the direct transformation of propylene oxide from propylene and hydrogen peroxide. SN - 3070-1058 PB - Institute of Central Computation and Knowledge LA - English ER -
@article{Pandey2025Oxygen,
author = {Ankit Pandey and Monojit Nandi and Gaurav Dwivedi and Ajay Singh and Samir Kumar Maity and Mukesh Kumar Poddar},
title = {Oxygen Insertion in Propylene to Make Propylene Oxide Over a Highly Stable and Efficient Titanium Silicate Catalyst},
journal = {Journal of Chemical Engineering and Renewable Fuels},
year = {2025},
volume = {2},
number = {1},
pages = {13-22},
doi = {10.62762/JCERF.2025.147821},
url = {https://www.icck.org/article/abs/JCERF.2025.147821},
abstract = {Propylene oxide (PO) is a crucial intermediate in the chemical industry, serving as a precursor for polyether polyols, propylene glycol, and various polymers. Traditional industrial PO production methods, such as the chlorohydrin and hydroperoxide routes, are hampered by significant environmental concerns and the generation of undesirable by-products. This study explores the direct transformation of propylene to PO using titanium silicate (TS-1) catalyst. The catalyst (TS-1) was synthesized and characterized by BET surface analysis, X-ray diffraction (XRD), scanning and transmission electron microscopy (SEM/TEM), and X-ray photoelectron spectroscopy (XPS). These analyses confirmed the formation of a crystalline MFI-type framework with well-dispersed titanium sites and a uniform, spherical morphology. Catalytic performance was evaluated in a batch reactor under varying conditions of temperature, pressure, hydrogen peroxide, and methanol concentrations. Under varying conditions, a maximum PO selectivity of 99.5\% and a PO yield of up to 9\% were achieved. The highest selectivity was obtained at lower H2O2 concentration, while the maximum yield required a higher H2O2 loading. The study also demonstrated the catalyst’s reusability and stability over multiple cycles, with minimal loss in activity. Side reactions, primarily the formation of propylene glycol and its ethers, were minimized by controlling water and hydrogen peroxide concentrations. The results highlight the advantages of TS-1, including high selectivity, environmental compatibility, and operational stability, making it a promising candidate for sustainable PO synthesis. This work provides valuable insights into the design of advanced heterogeneous catalysts for green chemical processes and addresses key challenges in the direct transformation of propylene oxide from propylene and hydrogen peroxide.},
keywords = {propylene, propylene oxide, TS-1, epoxidation},
issn = {3070-1058},
publisher = {Institute of Central Computation and Knowledge}
}
 Copyright © 2025 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
Copyright © 2025 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. Journal of Chemical Engineering and Renewable Fuels
ISSN: 3070-1058 (Online)
Email: [email protected]

Portico
All published articles are preserved here permanently:
https://www.portico.org/publishers/icck/