Journal of Plant Electrobiology
ISSN: pending (Online)
Email: [email protected]
 Submit Manuscript
Edit a Special Issue
Submit Manuscript
Edit a Special Issue
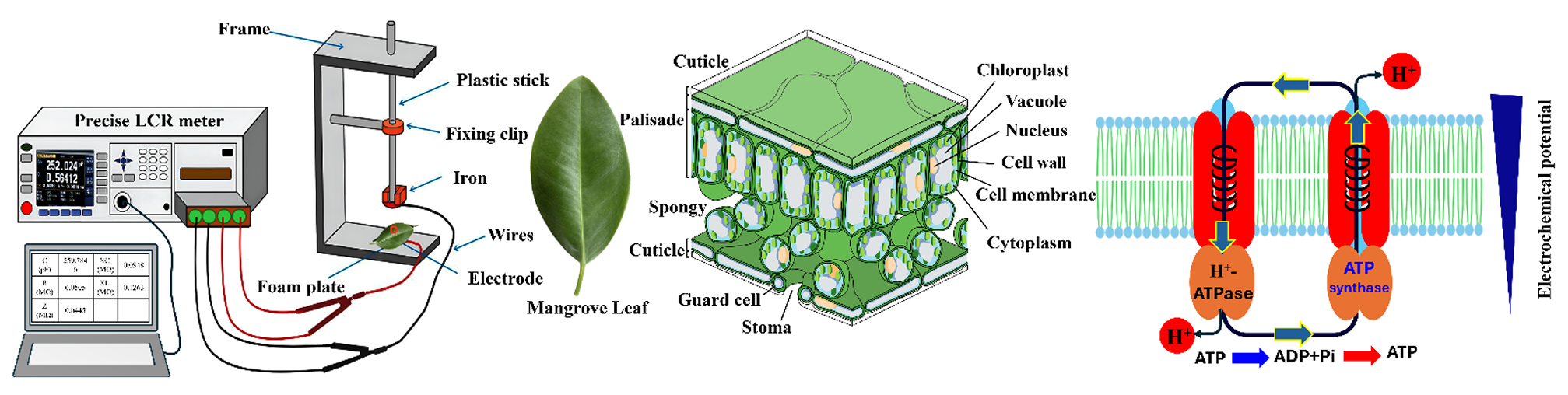
TY - JOUR AU - Wang, Jing AU - Aboueldahab, Mohamed AU - Wu, Yanyou AU - Xing, Deke AU - Zhang, Qian PY - 2026 DA - 2026/02/12 TI - Salt Adaptation in Aegiceras Corniculatum: Electrophysiology, Gene Expression, and Energy Trade-Offs JO - Journal of Plant Electrobiology T2 - Journal of Plant Electrobiology JF - Journal of Plant Electrobiology VL - 1 IS - 1 SP - 7 EP - 31 DO - 10.62762/JPE.2025.184208 UR - https://www.icck.org/article/abs/JPE.2025.184208 KW - aegiceras corniculatum KW - salt active transport KW - internal energy KW - photosynthesis KW - climate change AB - The integration of physical and chemical processes underpins life. Plant cells function as bioelectrical units, storing and converting energy through capacitive, inductive, and resistive properties. This study elucidates the electrophysiological and molecular mechanisms governing salt transport and energy allocation in Aegiceras corniculatum leaves under combined salinity-waterlogging stress (T1: 0.1 M NaCl + 2 h; T2: 0.2 M NaCl + 4 h; T3: 0.4 M NaCl + 6 h). Results demonstrate that leaf intracellular water-salt transport dynamics, coupled with salt-transport gene expression, coordinately regulate active/passive transport, vacuolar compartmentalization, cytoplasmic Na+ levels, and excretion. High salinity reduced salt excretion rate/capacity (LISTR/LISTC) and downregulated SOS1, while impairing water-holding capacity (LIWHC) and transport activities. Concurrent VHAc1 upregulation elevated vacuolar H+, inhibiting the Na+/H+ antiporter and compromising vacuolar salt sequestration. With increasing stress intensity, energy allocation shifted toward stress responses. Both electrical (internal) energy and ATP-derived chemical energy---originating from photosynthesis---jointly sustain plant vitality and adaptability; growth is primarily supported by internal energy, and adaptive differences dictate photosynthetic performance. This integrated analysis reveals how water-salt dynamics and molecular regulation confer salt tolerance in mangroves, offering insights crucial for coastal ecosystem resilience. SN - pending PB - Institute of Central Computation and Knowledge LA - English ER -
@article{Wang2026Salt,
author = {Jing Wang and Mohamed Aboueldahab and Yanyou Wu and Deke Xing and Qian Zhang},
title = {Salt Adaptation in Aegiceras Corniculatum: Electrophysiology, Gene Expression, and Energy Trade-Offs},
journal = {Journal of Plant Electrobiology},
year = {2026},
volume = {1},
number = {1},
pages = {7-31},
doi = {10.62762/JPE.2025.184208},
url = {https://www.icck.org/article/abs/JPE.2025.184208},
abstract = {The integration of physical and chemical processes underpins life. Plant cells function as bioelectrical units, storing and converting energy through capacitive, inductive, and resistive properties. This study elucidates the electrophysiological and molecular mechanisms governing salt transport and energy allocation in Aegiceras corniculatum leaves under combined salinity-waterlogging stress (T1: 0.1 M NaCl + 2 h; T2: 0.2 M NaCl + 4 h; T3: 0.4 M NaCl + 6 h). Results demonstrate that leaf intracellular water-salt transport dynamics, coupled with salt-transport gene expression, coordinately regulate active/passive transport, vacuolar compartmentalization, cytoplasmic Na+ levels, and excretion. High salinity reduced salt excretion rate/capacity (LISTR/LISTC) and downregulated SOS1, while impairing water-holding capacity (LIWHC) and transport activities. Concurrent VHAc1 upregulation elevated vacuolar H+, inhibiting the Na+/H+ antiporter and compromising vacuolar salt sequestration. With increasing stress intensity, energy allocation shifted toward stress responses. Both electrical (internal) energy and ATP-derived chemical energy---originating from photosynthesis---jointly sustain plant vitality and adaptability; growth is primarily supported by internal energy, and adaptive differences dictate photosynthetic performance. This integrated analysis reveals how water-salt dynamics and molecular regulation confer salt tolerance in mangroves, offering insights crucial for coastal ecosystem resilience.},
keywords = {aegiceras corniculatum, salt active transport, internal energy, photosynthesis, climate change},
issn = {pending},
publisher = {Institute of Central Computation and Knowledge}
}

Portico
All published articles are preserved here permanently:
https://www.portico.org/publishers/icck/