Oncology Communications
ISSN: pending (Online)
Email: [email protected]
 Submit Manuscript
Edit a Special Issue
Submit Manuscript
Edit a Special Issue
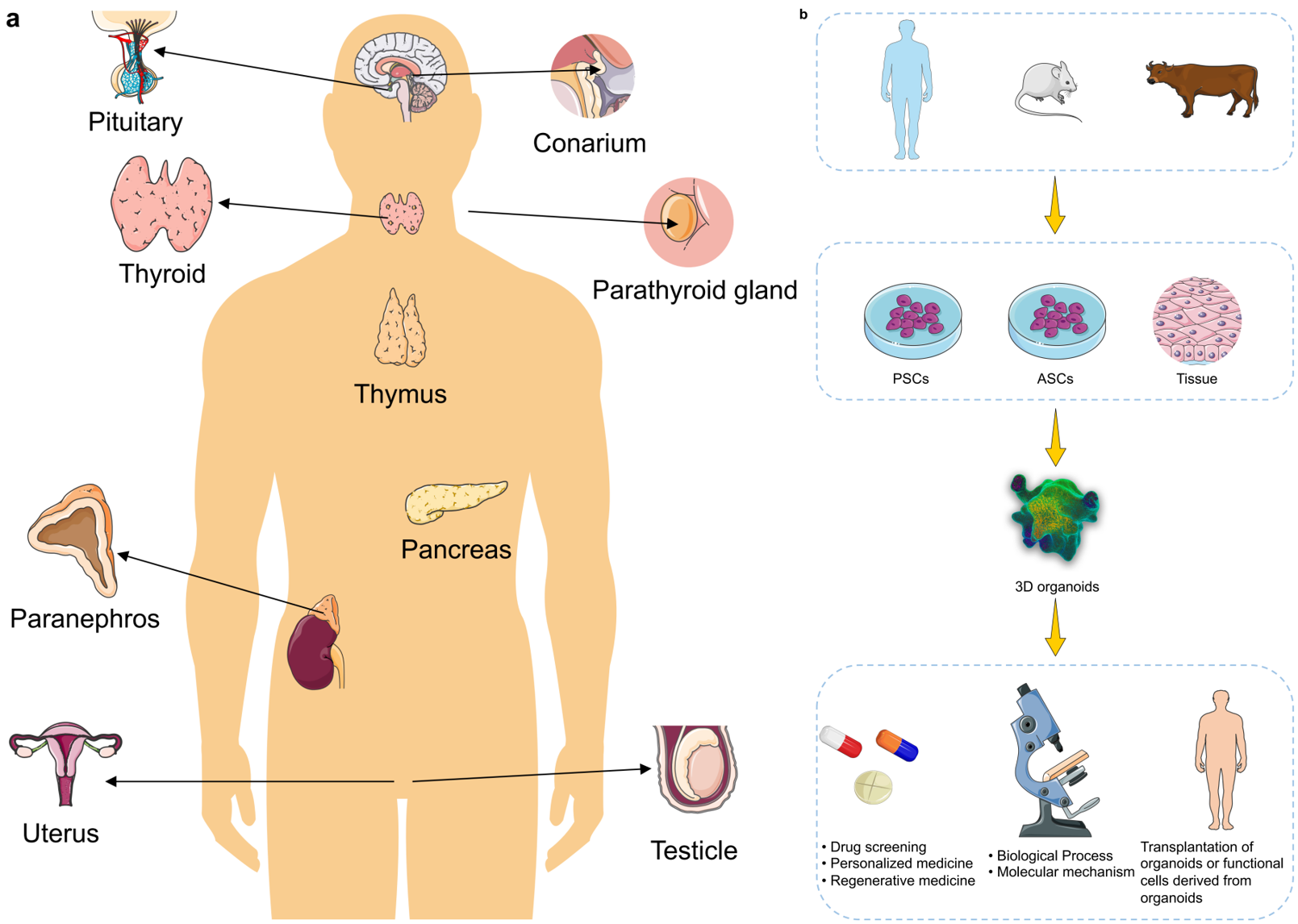
TY - JOUR AU - Liu, Jiayu AU - Li, Peiting PY - 2026 DA - 2026/02/06 TI - The Use of Organoid Models of Endocrine Diseases: Research Progress and Potential JO - Oncology Communications T2 - Oncology Communications JF - Oncology Communications VL - 1 IS - 1 SP - 8 EP - 19 DO - 10.62762/OC.2025.777438 UR - https://www.icck.org/article/abs/OC.2025.777438 KW - organoids KW - 3D culture KW - endocrine diseases KW - translational applications KW - disease modeling KW - tumor AB - The absence of robust and reliable $in \, vitro$ models that can accurately recapitulate the biological characteristics of many mammalian tissues and disease states represents a major barrier to both basic and translational research, owing to limited sample availability and ethical concerns. Stem cell-derived self-assembling three-dimensional (3D) organoids can replicate key structural and functional aspects of organs in a more physiologically relevant manner than traditional 2D models, thus providing a superior platform for simulating human physiology and pathology. To date, researchers have developed organoid models for a variety of endocrine tissues and their associated diseases (including pancreatic, pituitary, thyroid, adrenal tumors, etc.), offering invaluable tools for studying complex endocrine disorders. Such organoid models have significantly enhanced the accuracy and translational potential of research in disease modeling, drug screening, and regenerative medicine. Looking forward, the integration of bioengineering and multi-omics analyses with next-generation organoid models holds great promise for unraveling disease mechanisms and advancing precision medicine. SN - pending PB - Institute of Central Computation and Knowledge LA - English ER -
@article{Liu2026The,
author = {Jiayu Liu and Peiting Li},
title = {The Use of Organoid Models of Endocrine Diseases: Research Progress and Potential},
journal = {Oncology Communications},
year = {2026},
volume = {1},
number = {1},
pages = {8-19},
doi = {10.62762/OC.2025.777438},
url = {https://www.icck.org/article/abs/OC.2025.777438},
abstract = {The absence of robust and reliable \$in \, vitro\$ models that can accurately recapitulate the biological characteristics of many mammalian tissues and disease states represents a major barrier to both basic and translational research, owing to limited sample availability and ethical concerns. Stem cell-derived self-assembling three-dimensional (3D) organoids can replicate key structural and functional aspects of organs in a more physiologically relevant manner than traditional 2D models, thus providing a superior platform for simulating human physiology and pathology. To date, researchers have developed organoid models for a variety of endocrine tissues and their associated diseases (including pancreatic, pituitary, thyroid, adrenal tumors, etc.), offering invaluable tools for studying complex endocrine disorders. Such organoid models have significantly enhanced the accuracy and translational potential of research in disease modeling, drug screening, and regenerative medicine. Looking forward, the integration of bioengineering and multi-omics analyses with next-generation organoid models holds great promise for unraveling disease mechanisms and advancing precision medicine.},
keywords = {organoids, 3D culture, endocrine diseases, translational applications, disease modeling, tumor},
issn = {pending},
publisher = {Institute of Central Computation and Knowledge}
}
 Copyright © 2026 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
Copyright © 2026 by the Author(s). Published by Institute of Central Computation and Knowledge. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. 
Portico
All published articles are preserved here permanently:
https://www.portico.org/publishers/icck/